When you think about it, what other choice is there but to hope?
We have two options, medically and emotionally: give up, or fight like hell.
Lance Armstrong
Hippocrates was one of the first to describe different kinds of cancers, using the word oncos(Greek for “swelling”) to describe benign tumours, and carcinos (Greek for “crab”) for malignant tumours possibly due to the appearance of the cut surface of some solid malignant tumours which vaguely resembles the silhouette of a crab. However, he was certainly not the first to discover the disease and the documentary history of cancer stretches back to 1500 BC in ancient Egypt where a papyrus describes how breast cancers were treated by using a hot piece of metal to burn away the tissue.
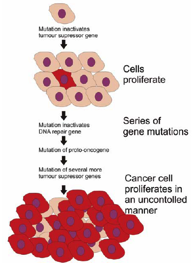
Cancer basically describes an uncontrolled division of cells in which malignant cells can either invade adjacent tissues or else implant themselves into distant sites (known as metastasis). This unregulated cell growth is caused by damage to DNA, resulting in mutations to specific genes coding for proteins regulating cell growth and division, known as proto-oncogenes and tumour suppressor genes. Proto-oncogenes promote cell growth in a variety of ways by coding for proteins such as hormones, signal receptors, or transcription factor proteins which can increase expression of other genes. Tumour suppressor genes, on the other hand, code for anti-proliferation signals and proteins that suppress mitosis and cell growth. These tumour suppressors are normally activated by cell stress or DNA damage, thereby serving to halt cell division in order to carry out DNA repair, preventing mutations from being passed on to daughter cells. One example of a tumour suppressor is the p53 protein, which is activated by many cellular stressors including low oxygen and UV radiation damage. A mutation damaging the gene for p53, or in a gene controlling p53, can result in a lack of this protein with the consequence that DNA repair is inhibited, allowing further DNA damage to accumulate, inevitably leading to cancer. However, mutations in several of these genes, resulting in a loss of a tumour suppressor protein or in an increasing level of a proto-oncogene protein, are required before a normal cell transforms into a cancer cell; a mutation limited to only one gene would be suppressed by the control of normally functioning tumour suppressor genes.
Familial cancer syndromes result from the inheritance of a mutation in a tumour suppressor or proto-oncogene gene, increasing the chance of developing particular cancers. Mutations in tumour suppressor genes generally act in a recessive manner with a mutation in the second, normally functioning, copy of the gene needed before a cell transforms into a cancer cell. For instance, individuals who are heterozygous for p53 mutations are often victims of Li-Fraumeni syndrome. As long as one copy of the p53 gene produces a functional protein, tumours do not form. However, if a mutation occurs in the second copy of the gene during a person’s lifetime, the cell will have no working copies of the gene and will produce no functional protein allowing tumours to develop. This also occurs in mutations in the retinoblastoma gene increasing the chance of developing cancer of the retina, mutations in BRCA1 or BRCA2 leading to early onset of breast cancer and von Hippel-Lindau syndromedue to mutations of the Von Hippel-Lindau tumour suppressor gene.
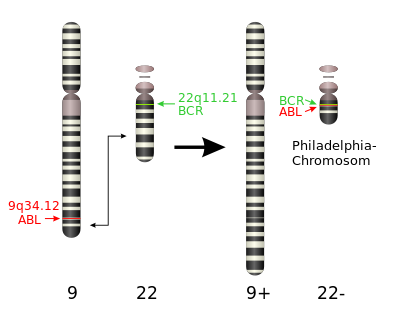
Proto-oncogenes are gene involved in normal cell growth that mutations in may cause it to become an oncogene, which can cause the growth of cancer cells. An example would be the 2 protooncogenes (BCR and ABL) that are swapped and joined from 2 different chromosomes to form fused gene known as BCR-AB with the errant chromosome known as a Philadelphia chromosome leading to chronic myeloid leukemia.
Of all cases of cancer, only a small percent derive from a hereditary predisposition, with most cancers occurring sporadically and being linked to whole range of factors including environment and life style. Therefore, the presence of one or two cases of cancer in a family does not necessarily mean an increased genetic risk. Many factors can help determine if the cancer in a family is hereditary, including the presence of certain types of cancer occurring in the same family (for example breast cancer and ovarian cancer, or colon cancer and uterine cancer), the age of onset of cancer (particularly for breast cancer and colon cancer, onset before age 50 is considered more significant), and the number of relatives with cancer and their relationship to each other.
Li-Fraumeni Syndrome
Autosomal dominant
Mutations in the p53 gene can lead to Li-Fraumeni syndrome, characterised by an increased chance of cancers in a variety of different tissues such as breast cancer, brain tumors, acute leukemia, soft tissue sarcomas, bone sarcomas, or adrenal cortical carcinoma if cells here acquire mutations in the second copy of the p53 gene.
Reza Dehghani, founder of Criminal Clothing, died aged 32 in 2008, having suffered from cancer caused by Li-Fraumeni syndrome. Neither his grandmother, his mother’s sister, or his own sister, lived through their 30s.
BRCA1 and BRCA2
The BRCA1 and BRCA2 genes are tumor suppressor genes producing proteins involved in repairing damaged DNA. These breaks can be caused by natural and medical radiation or other environmental exposures, and also occur when chromosomes exchange genetic material in preparation for cell division. By helping repair DNA, these proteins maintain the stability of a cell’s genetic information. Mutations in BRCA1 and BRCA2 can lead to early onset of breast and ovarian cancer.
In 2008 actress Christina Applegate had both breasts removed in an effort to prevent her breast cancer from returning. This was after a test that showed that she had inherited a mutant BRCA1 gene from her mother who suffered breast cancer and cervical cancer.

Von Hippel-Lindau Syndrome

Von Hippel-Lindau syndrome occurs due to mutations of the Von Hippel-Lindau tumour suppressor gene resulting in the growth of tumours in various parts of the body such as in the central nervous system, and particularly in the adrenal gland.
It has been suggested that the hostility underlying the famous American Hatfield-McCoy feud of the late 1800s may have been partly due to the consequences of Von Hippel-Lindau disease. Generations of the two families fought often deadly battles over land, timber rights and even a pig and are the subject of dozens of books songs and countless jokes. It seems that the McCoy family was pre-disposed to bad tempers partly because many of them had adrenal gland tumours which even now many of their descendents still suffer from. This tumour leads to increased production of adrenaline causing a tendency toward explosive tempers in addition to high blood pressure, pounding headaches, heart palpitations, facial flushing, nausea and vomiting.
Philadelphia Chromosome
Philadelphia translocation is a specific chromosomal abnormality where parts of chromosomes 9 and 22 swap places resulting in the formation of a fused gene. This new abnormal gene interferes with the cell cycle speeding up cell division an disrupting the repair of DNA damage.
This consequently results in an increased chance of developing chronic myelogenous leukemia (CML).

Kareem Abdul-Jabbar the retired American basketball player, coach, actor, and author announced that he was suffering from Philadelphia chromosome-positive chronic myeloid leukemia in 2009.
